Cell Phenotyping
To date, cell state and function has primarily been probed by investigating biochemical properties. However intrinsic biophysical markers, such as cell deformability under a load, offer extended information about the cell while not necessarily requiring costly labeling or sample preparation (Gossett et al. 2012, PNAS 109: 7630 ). Cell mechanics, specifically, has been shown to be highly a sensitive marker in biology - as an indicator for a particular state or pathology (Dupont et al. 2011, Nature 474: 179 ).
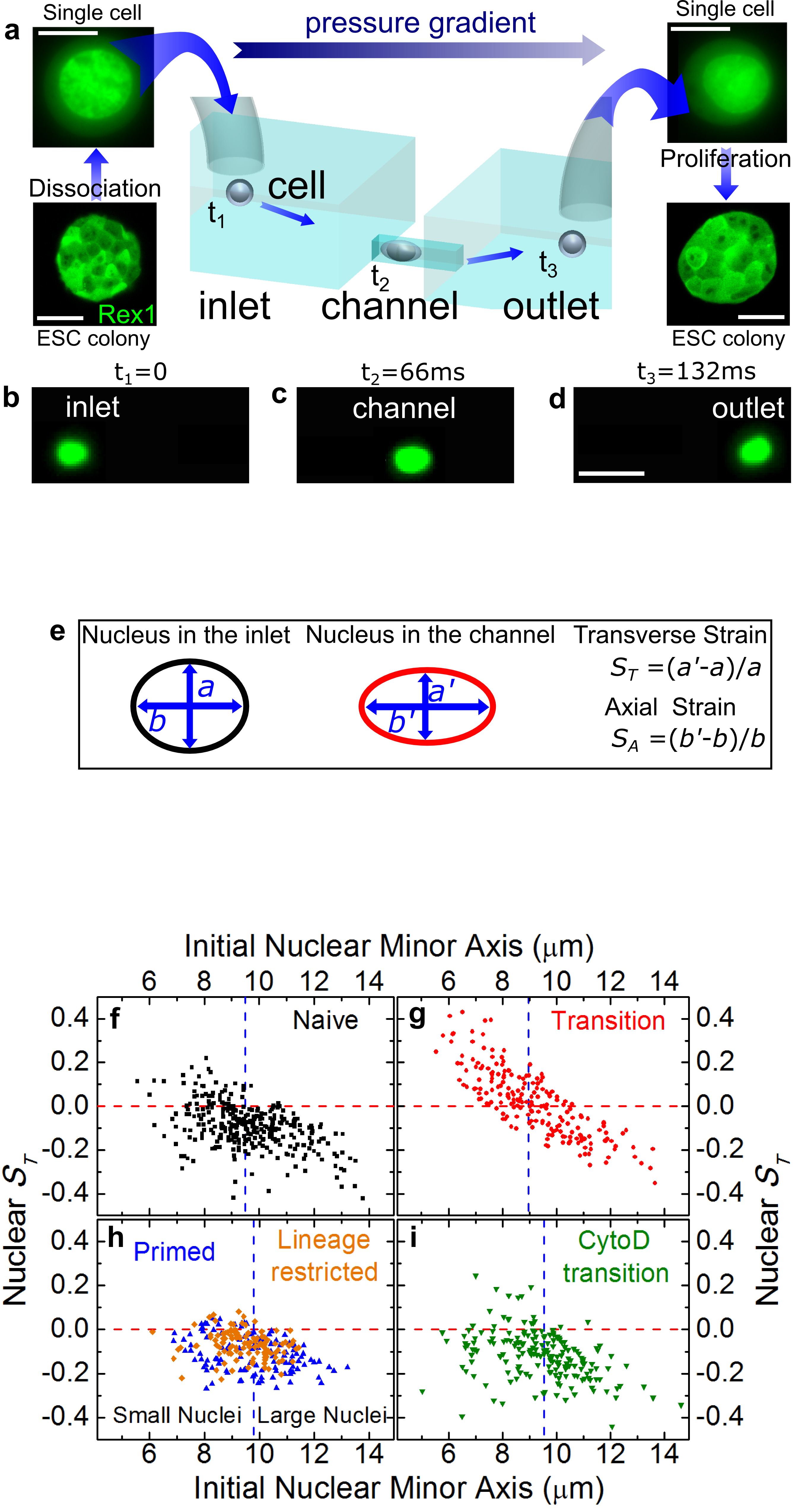
We developed a microfluidic platform to advect live cells across confined channels by using a pressure differential (Hodgson et al. 2017, Lab on a Chip 17: 805). The cells can be taken from culture, advected and confined through the microfluidics as illustrated in Figure a-d.
By using this technology we show that during the course of differentiation of human myeloid precursor cells into three different lineages, the cells alter their viscoelastic properties, to suit their ultimate fate and function. Myeloid cells circulating in blood have to be advected through constrictions in blood vessels, engendering the need for compliance at short time-scales (less than a second). Intriguingly, only the two circulating myeloid cell types have increased short time scale compliance and flow better through microfluidic constrictions (Ekpenyong et al. 2012, PLoS One 7: e45237).
Furthermore, by using this technology we also investigate embryonic stem cells that self-renew in a state of naive pluripotency in which they are competent to generate all somatic cells. It has been hypothesized that, before irreversibly committing, they pass through at least one metastable transition state, representing a gateway for differentiation and reprogramming of somatic cells. We show that during the transition, the nuclei of embryonic stem cells are auxetic: they exhibit a cross-sectional expansion when stretched (Figure e-i) and a cross-sectional contraction when compressed, and their stiffness increases under compression (Pagliara et al. 2014, Nature Materials 13: 638). Through the regulation of molecular turnover in the differentiating nucleus by external forces, auxeticity could be a key element in mechanotransduction. Our findings highlight the importance of nuclear structure in the regulation of differentiation and reprogramming.
In order to increase the throughput of our method, in collaboration with the Guck lab we recently introduced real-time deformability cytometry (RT-DC) for continuous mechanical single-cell characterization on-the-fly with analysis rates in excess of 100 cells/s (Otto et al. 2015, Nature Methods 12: 199).
In collaboration with Richard Chahwan, Nick Stone and Francesca Palombo we are currently further developing these technologies to investigate immune system cells.
News
- 8-September-2023: Congratulations Erin! New paper out at ISME Communications
- 10-April-2023: Congratulations Ula! New paper out at PLoS Biology
- 7-June-2022: Congratulations Ula! New paper out at eLife

