Food allergy continues to rise in the US and Europe with peanut allergy suffers producing the largest group of allergy-related fatalities (1-4). In the US as many as 2% of children show peanut allergy, often compounded with multiple allergies, leading to a disproportionately large number of deaths (5) and prompting some scientists to consider an approaching peanut allergy epidemic (6). The exact cause of developing a peanut allergy is unknown (5,7). There is evidence for and against ingestion of peanut during pregnancy correlating with subsequent infant peanut allergy (8). Furthermore, delaying the introduction of peanuts to young children has been shown to increase the risk of developing an allergy to peanuts (9). It is also possible that exposure to peanut oils in lotions and creams may be implicated in the development of this allergy (8). This is supported by a recent study that has demonstrated a significant association between mutations in the fillagrin gene, which plays a major role in the epithelial barrier, and the risk of developing peanut allergy (10).
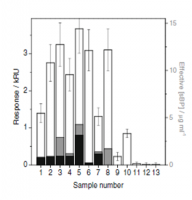
We have recently performed a new screen of serum of 14 peanut allergy patients and using the label-free array reader technology that can detect anything related to peanut allergy in the blood. The conventional marker is IgE and this is measured in the hospital routinely; secondly there is IgG which can also be measured but this does not happen frequently; thirdly there is something else. Looking at Figure 1, the total response of a patient’s blood to peanut proteins is made up from the three contributions: IgE, IgG and our new ara Factor. For many of the patients, the concentration of the ara Factor is bigger than the other two components, Figure 2.
Aims and Objectives
The new ara Factor may not be important but it is at least different and may offer a way to understand how patients grow out of allergy. The ara Factor may be protective, preventing the IgE from binding to the protein and causing the allergic response.
Next Steps:
- Identify the ara Factor from a number of patients to determine whether it is a known protein or whether it is something completely new.
- Look at the blood of a larger group of peanut allergy sufferers, probably 150, to see how important the new ara Factor is.
- Look at the blood of people that have grown out of peanut allergy to see if the ara factor is protective.
We need to extent this new screen to other food allergies and to see of people that develop gluten allergy rapidly have the any new components. Similarly, is there a link with irritable bowel disease?
Funding applications have been filed and if successful, we will need to recruit a large patient cohort.
References
1. Sicherer, S. H., and Sampson, H. A. (2010) Journal of Allergy and Clinical Immunology 125, S116-S125
2. Sicherer, S. H., Muñoz-Furlong, A., Godbold, J. H., and Sampson, H. A. (2010) Journal of Allergy and Clinical Immunology 125, 1322-1326
3. Bock, S. A., Munoz-Furlong, A., and Sampson, H. A. (2007) Journal of Allergy and Clinical Immunology 119, 1016-1018
4. Eigenmann, P. A., and Zamora, S. A. (2002) Allergy 57, 449-453
5. Hourihane, J. O. B. (2011) Pediatric Clinics of North America 58, 445-458
6. Sicherer, S. H., and Sampson, H. A. (2007) Journal of Allergy and Clinical Immunology 120, 491-503
7. Husain, Z., and Schwartz, R. A. Journal of the American Academy of Dermatology In Press, Corrected Proof
8. Lack, G., Fox, D., Northstone, K., and Golding, J. (2003) New England Journal of Medicine 348, 977-985
9. Du Toit, G., Katz, Y., Sasieni, P., Mesher, D., Maleki, S. J., Fisher, H. R., Fox, A. T., Turcanu, V., Amir, T., Zadik-Mnuhin, G., Cohen, A., Livne, I., and Lack, G. (2008) Journal of Allergy and Clinical Immunology 122, 984-991
10. Weidinger, S., Illig, T., Baurecht, H., Irvine, A. D., Rodriguez, E., Diaz-Lacava, A., Klopp, N., Wagenpfeil, S., Zhao, Y., Liao, H., Lee, S. P., Palmer, C. N. A., Jenneck, C., Maintz, L., Hagemann, T., Behrendt, H., Ring, J., Nothen, M. M., McLean, W. H. I., and Novak, N. (2006) Journal of Allergy and Clinical Immunology 118, 214-219