|
||||
|---|---|---|---|---|
The process of oxidisation and supergene enrichment has lead not only the exploitation of rich ore but has also produced a very large variety of minerals, sometimes rare, in the orefield. Oxidation of ore bearing rocks continues today and new minerals are being formed in mine waste dumps or in old mine workings.
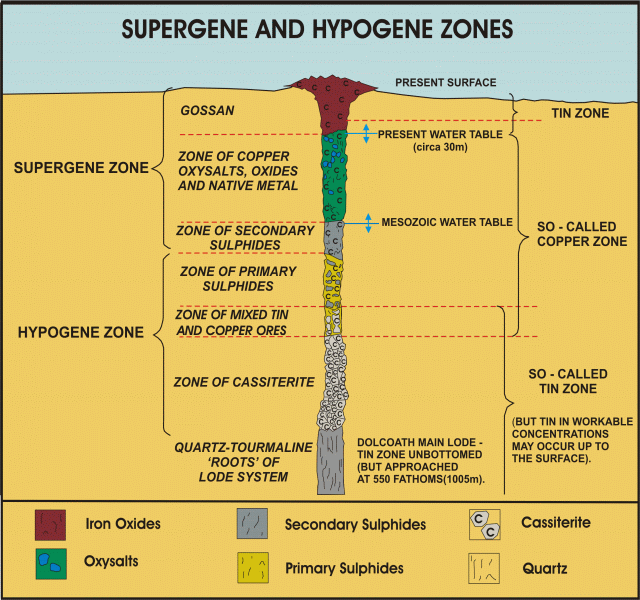
In the Cornibian Orefield, early hardrock mining of cassiterite was from gossan ores. These were enriched in tin values due to the oxidization and consequent removal of the sulphides, leaving only cassiterite with gangue minerals of iron oxides and quartz. Below the gossan, rich copper ores were encountered leading to a period of copper mining which mainly ignored the tin values. Later as these were exhausted at depth the unoxidized ore, or proto-ore, was encountered with both tin and copper mining taking place in the mixed ore. With depth, the sulphide content decreases with only cassiterite and silicate gangue minerals remaining. |
||||
After Hosking, 1988. |
||||
This site was last updated on
Friday, December 12, 2003 1:09 PM
.
Webmasters : Simon Camm & Paul
Hedley