VII.4 Combustion modelling - direct approach
Since combustion is basically a chemical reaction process combining reactants to form
products, a direct approach to modelling it would be to work with the individual components.
We can write down balance equations for the various reactants and the energy equation,
together as usual with the continuity and momentum equations, and solve the entire system to
calculate the flow. The quantity of chemical species i present at any point in the flow is
indexed by the mass fraction Y i - the mass of the species per unit mass of the mixture (eg. kg
of species i per kg of gas). A transport equation can be written for each Y i in the system
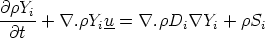 | (VII.1) |
where Di is some diffusivity, and of course
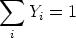 The source term Si accounts for the addition or removal of species i due to the combustion
processes. At the same time an energy equation must be solved. This can be an equation for
the internal energy e, or equivalently for the enthalphy h (automatically taking into account
the pdV work being done in expansion) or the temperature T . For example, a balance equation
for T can be formulated
The source term Si accounts for the addition or removal of species i due to the combustion
processes. At the same time an energy equation must be solved. This can be an equation for
the internal energy e, or equivalently for the enthalphy h (automatically taking into account
the pdV work being done in expansion) or the temperature T . For example, a balance equation
for T can be formulated
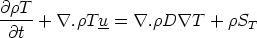 | (VII.2) |
The source term here includes such effects as radiation loss or gain, pressure work as well as
the chemical energy release due to the combustion. All the equations describing the reacting
components are very similar in form, and for notational simplicity are sometimes grouped into
a vector denoting “reactive scalars”
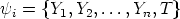 | (VII.3) |
(for n species in the reaction), with a governing transport equation of the form
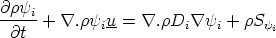 | (VII.4) |
There are a number of problems with doing this however. Firstly, in a complete
system n is probably very large : to make this a realistic model we need to reduce its
complexity somehow. Also we have not yet discussed the effects of turbulence on the
flow.
A great deal of information has been collected about the chemical reactions occurring in
various important combustion processes. Significant data includes the rates of reaction kf and
kb for the forward and backward reaction processes and the stoichiometric coefficients for the
reactions. From this information the heat release from the reactions, and ultimately the source
terms S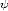 i, can be determined. This information can be combined into tables called elementary
reaction mechanisms which describe in detail the chemical reactions proceeding during
combustion. These are extensively used when the detail of the chemical processes is important,
but as they often take into account hundreds of individual reactions, and are frequently
numerically ‘stiff’ (ie. difficult to solve), they are difficult to incorporate into a full
fluid flow/combustion model. To develop such a model it is necessary to simplify
the processes involved whilst at the same time without losing the more important
details.
i, can be determined. This information can be combined into tables called elementary
reaction mechanisms which describe in detail the chemical reactions proceeding during
combustion. These are extensively used when the detail of the chemical processes is important,
but as they often take into account hundreds of individual reactions, and are frequently
numerically ‘stiff’ (ie. difficult to solve), they are difficult to incorporate into a full
fluid flow/combustion model. To develop such a model it is necessary to simplify
the processes involved whilst at the same time without losing the more important
details.
Hydrocarbon combustion consists of chain reactions of the general form
| A + B |  C C | |
|
| C + D |  E E | |
|
| E + F |  G G | |
|
|  | |
|
| N + O |  P P | | |
The intermediate steps of the process A + B  P often involve short-lived radical species
which remain in quasi-steady state throughout the process : these are fast reactions which
proceed rapidly. If these can be eliminated (the Quasi-Steady-State Assumption, QSSA) then
the reaction can be effectively represented by a reduced set of slower, rate-determining
reactions, called a global reaction mechanism. For example, an elementary reaction
mechanism for methane oxidation might detail 277 reactions involving 49 chemical species
but is frequently reduced down to a global reaction mechanism involving just 4
steps
P often involve short-lived radical species
which remain in quasi-steady state throughout the process : these are fast reactions which
proceed rapidly. If these can be eliminated (the Quasi-Steady-State Assumption, QSSA) then
the reaction can be effectively represented by a reduced set of slower, rate-determining
reactions, called a global reaction mechanism. For example, an elementary reaction
mechanism for methane oxidation might detail 277 reactions involving 49 chemical species
but is frequently reduced down to a global reaction mechanism involving just 4
steps
| I | CH4 + 2H + H2O | = CO + 4H2 | | | |
|
| II | CO + H2O | = CO2 + H2 | | | |
|
| III | H + H + M | = H2 + M | | | |
|
| IV | O2 + 3H2 | = 2H + 2H2O | | | | |
![]() i, can be determined. This information can be combined into tables called elementary
reaction mechanisms which describe in detail the chemical reactions proceeding during
combustion. These are extensively used when the detail of the chemical processes is important,
but as they often take into account hundreds of individual reactions, and are frequently
numerically ‘stiff’ (ie. difficult to solve), they are difficult to incorporate into a full
fluid flow/combustion model. To develop such a model it is necessary to simplify
the processes involved whilst at the same time without losing the more important
details.
i, can be determined. This information can be combined into tables called elementary
reaction mechanisms which describe in detail the chemical reactions proceeding during
combustion. These are extensively used when the detail of the chemical processes is important,
but as they often take into account hundreds of individual reactions, and are frequently
numerically ‘stiff’ (ie. difficult to solve), they are difficult to incorporate into a full
fluid flow/combustion model. To develop such a model it is necessary to simplify
the processes involved whilst at the same time without losing the more important
details.