X-ray fluorescence spectrometry is a relatively quick and effective way to measure major oxide and trace element abundances in powdered whole rock samples. The main principle behind XRF spectrometry is that X-rays of characteristic wavelength (and energy) are emitted from a sample when the sample is ionised by a stream of X-rays; this process is known as X-ray fluorescence.
Most elements between atomic number 11 and 92 (e.g. Na to U) are routinely measured. There are two main sample preparation methods used for analysis; light elements and major oxides are very susceptible to mineralogical (matrix) effects and must be fused into a glass disk (fused bead); heavier elements are more straight-forward and samples can be pressed into pellets.
Instrumentation
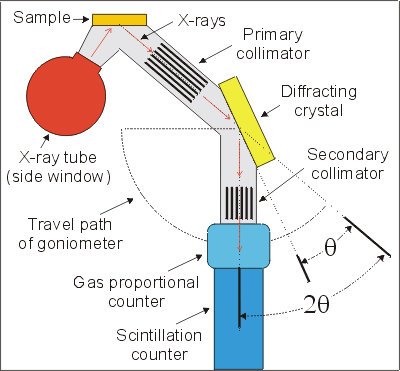
X-ray fluorescence is relatively simple and comprises two components: an X-ray source, and (in most applications) a wavelength dispersive spectrometer (WDS).
The basic principles behind wavelength dispersive spectrometers are that X-rays with a wavelength (l) are only reflected by a crystal lattice when the distance between planes of atoms (d) and the angle of incidence (q) satisfy Bragg's law:
nl=2dsinq
where n is an integer.
As the d value of the diffracting crystal is known, the detector and diffracting crystal can be moved (using the goniometer) through an angle q so that only X-rays with a specific wavelength arrive at the detectors. Therefore, the X-ray detector can be "tuned" to measure only the X-rays produced by fluorescence of atoms of one element; the intensity of this radiation is proportional to the abundance of that element within the sample.

A narrow beam of monochromatic X-rays is incident upon the sample which fluoresces to produce the characteristic X-rays derived from ionisation of the component atoms. The characteristic X-rays pass through the primary collimator to the diffracting crystal. Only X-rays with wavelengths satisfying Bragg's law are reflected by the crystal through to the X-ray counters where the intensity of the radiation is measured. The type of diffracting crystal and the angle of the goniometer is dependent on the element being analysed. Most of the functions of the XRF are controlled externally by a computer.
Optimal analyses
 XRF
is a relatively simple and easy method for analysing solid inorganic samples
(particularly geological samples). The technique is particularly well suited to
the determination of major elements within silicates. In
addition, XRF is a reliable method for trace element analyses at
the ppm level although the technique is relatively insensitive to the rare earth
elements (e.g. La, Ce etc). Typical
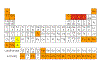
detection limits for XRF are summarised here.
XRF
is a relatively simple and easy method for analysing solid inorganic samples
(particularly geological samples). The technique is particularly well suited to
the determination of major elements within silicates. In
addition, XRF is a reliable method for trace element analyses at
the ppm level although the technique is relatively insensitive to the rare earth
elements (e.g. La, Ce etc). Typical
detection limits for XRF are summarised here.
Advantages
Relatively simple, cheap and quick analyses
Accurate analyses of a range of elements
"Dry" method and therefore requires minimal sample preparation (for trace element analysis).
Few consumables.
Disadvantages
Unsuitable for analysis of very light elements e.g. H to Ne.
Analysis of liquids requires the use of large volumes of He.
Fused bead preparation is time consuming and takes a certain amount of practice.